TargetMol中国品牌商
12 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
非小细胞肺癌MET抑制剂飞速发展,国产靶向药效果显著
915 人阅读发布时间:2020-08-24 17:42
尽管目前肺癌仍然是世界上致死率最高的肿瘤,但在具有某些特定基因突变的NSCLC 患者中,靶向治疗的出现使患者的预后有了较大的提升,近年来,MET 抑制剂在 MET 外显子跳跃突变的癌症治疗中得到了良好的表现,获得药物研发人员的广泛关注。
获批药物Tepotinib
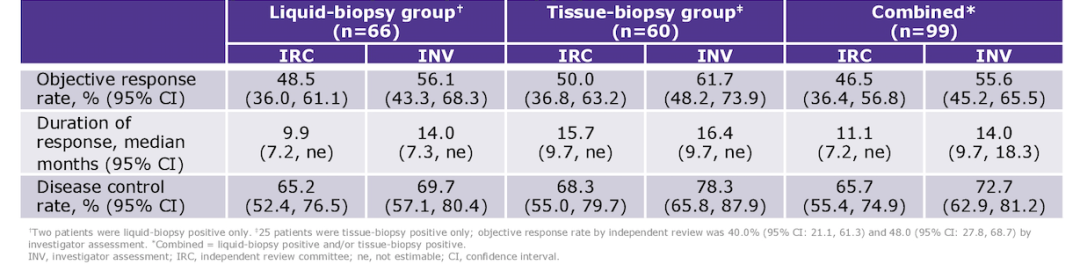
相关的 VISION 数据实验结果中,共有 152 例患者接受了 tepotinib 治疗,其中 99 例接受了至少 9 个月的随访。无论是液体活检 (Liquid组) 或组织活检 (Tissue组) Tepotinib 治疗疗效无显著差异。两组的 ORR 分别为 48.5%、50%,DoR 为 9.9 个月、15.7 个月,DCR 分别为 65.2%、68.3%。
FDA 批准 Tabrecta 应用于 NSCLC
国产药 savolitinib 上市申请纳入优先审评
| savolitinib | Tepotinib | Capmatinib | |
|---|---|---|---|
| PSC 比例 |
35.7% |
1.1% | 5% |
| 脑转移 比例 |
22% |
9.2% | 13.4% |
| DCR |
93.4% |
72.5% | 82% |
| ORR |
48% |
42% | 47% |
各抑制剂疗效对比
非小细胞肺癌 (NSCLC) 的靶向治疗在近年得到了白热化的发展,我们相信,随着医药研发的不断进步,会有越来越多的肺癌患者因此受益。
Targetmol 助力科研
编号:TQ0210
别名:Volitinib, AZD-6094
CAS:1313725-88-0
分子式:C17H15N9
编号:T6121
别名:EMD-1214063, MSC2156119
CAS:1100598-32-0
分子式:C29H28N6O2
靶点:AXL; c-Met; TrkA;
Capmatinib
编号:T1963
别名:INC-280, INCB28060, NVP-INC280
CAS:1029712-80-8
分子式:C23H17FN6O
靶点:c-Met;
抗肺癌化合物库
编号:L2190
产品特性:
-
400 种与肺癌相关的化合物,可以用于抗肺癌药物研发和药理研究;
-
靶点包含 EGFR, PI3K, FGFR, HER2, ALK, c-Met等;
-
部分化合物已上市或进入临床期;
-
详细的说明书,化合物结构、靶点信息、IC50 值、活性描述等;
-
NMR、HPLC/LCMS 等多种检测技术保证产品结构正确,纯度高,减少假阳性。
参考文献:
2. 2019 AACR、2020ASCO
3. Lecia V Sequist, Ji-Youn Han, Myung-Ju Ahn et al.Osimertinib plussavolitinib in patients with EGFR mutation-positive, MET-amplified,non-small-cell lung cancer after progression on EGFR tyrosine kinaseinhibitors: interim results from a multicentre, open-label, phase 1b study. TheLancet. 2020
4. Ibiayi Dagogo-Jack et al. MET Alterations are a Recurring andActionable Resistance 2 Mechanism in ALK-Positive Lung Cancer.2020
5. Acquired METD1228V Mutation and Resistance to MET Inhibition in LungCancer.2016
6. Landi, L. et al. Crizotinib in MET deregulated or ROS1 rearrangedpretreated non-small-cell lung cancer (METROS): a phase II, prospective,multicentre, two-arms trial. Clin. Cancer Res.
7. Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates ofincidence and mortality worldwide for 36 cancers in 185 countries. CACancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492PMID:30207593
8. Ferlay J, et al (2018). Global Cancer Observatory: Cancer Today. Lyon,France: International Agency for Research on Cancer. Accessed 20 March 2020
9. Salgia R. MET in lung cancer: Biomarker selection based on scientificrationale. Mol Cancer Ther. 2017;16(4):555–565. doi:10.1158/1535-7163.mct-16-0472.
10. Le X,Felip E, Veillon R, et al. Primary efficacy and biomarker analyses from theVISON study of tepotinib in patients (pts) with non-small cell lung cancer(NSCLC) with METex14 skipping. J Clin Oncol. 2020;38(suppl 15; abstr9556). doi:10.1200/JCO.2020.38.15_suppl.9556
11. Paik PK,Felip E, Veillon R, et al. Tepotinib in non–small cell lung cancer with METexon 14 skipping mutations. N Eng J Med. Published online May 29, 2020.doi:10.1056/NEJMoa2004407
12. MerckKGaA, Darmstadt, Germany, announces FDA Breakthrough Therapy Designation forinvestigational therapy tepotinib in patients with metastatic NSCLC withMETex14 skipping alterations. News release. Merck KGaA. Published September 11,2019. Accessed May 31, 2020. https://prn.to/2kuDROO
如有需要,可拨打400-821-2233随时联系我们,也可登录 Targetmol 官网查看产品和服务的更多信息。



